Neural Repair
- Projects
- Scientists/Team
- Publications
- BDNF Gene Therapy Trial in Alzheimer's Disease
- Giving
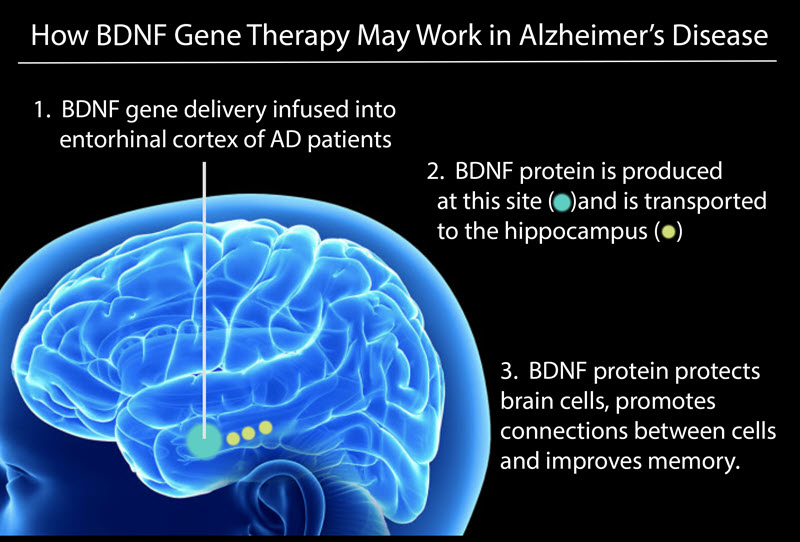
The Tuszynski group is sponsoring a first-in-human clinical trial of gene therapy in Alzheimer’s Disease and Mild Cognitive Impairment.
This Phase 1 clinical trial will examine whether the nervous system growth factor “Brain-Derived Neurotrophic Factor “(BDNF) will prevent neuronal loss and build new synapses, thereby potentially improving memory in patients with Alzheimer’s disease (AD) and Mild Cognitive Impairment (MCI).
This trial has been approved by the FDA and the UC San Diego Human Subjects Safety Committee.
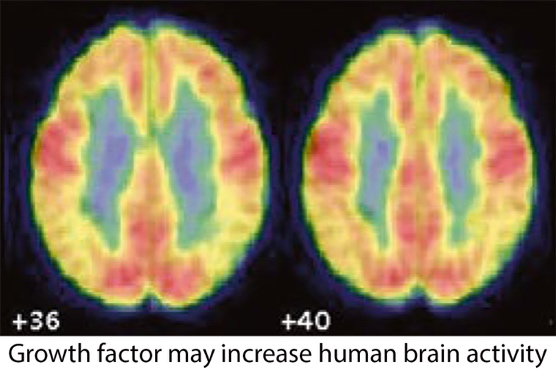
I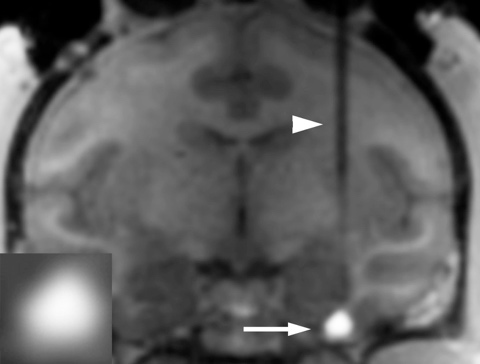 n animal studies that include “Alzheimer’s mice”, aged rats and aged rhesus monkeys, BDNF gene therapy prevented the death of cells in the brain and stimulated their functional state. This resulted in improvement in memory. This work was first published in Nature Medicine in 2009, and has been followed by several additional scientific reports.
n animal studies that include “Alzheimer’s mice”, aged rats and aged rhesus monkeys, BDNF gene therapy prevented the death of cells in the brain and stimulated their functional state. This resulted in improvement in memory. This work was first published in Nature Medicine in 2009, and has been followed by several additional scientific reports.
The BDNF gene will be delivered into the brain using harmless, natural viruses called “AAV2”. These have been used in many human clinical trials safely, including 6 clinical trials of brain disorders.
 Gene therapy has led to breakthroughs in the treatment of a hereditary neurological disorder in humans called Spinal Muscular Atrophy, and a hereditary cause of blindness in humans (Leber’s Optic Atrophy). We now aim to determine whether the promise of gene therapy will also benefit patients with Alzheimer’s disease and Mild Cognitive Impairment.
Gene therapy has led to breakthroughs in the treatment of a hereditary neurological disorder in humans called Spinal Muscular Atrophy, and a hereditary cause of blindness in humans (Leber’s Optic Atrophy). We now aim to determine whether the promise of gene therapy will also benefit patients with Alzheimer’s disease and Mild Cognitive Impairment.
Patients for this trial will be recruited at UC San Diego and at Case Western Reserve University in Cleveland, Ohio.
Nobuko Kemmotsu
nkemmotsu@health.ucsd.edu
858-246-1267
