Our Research
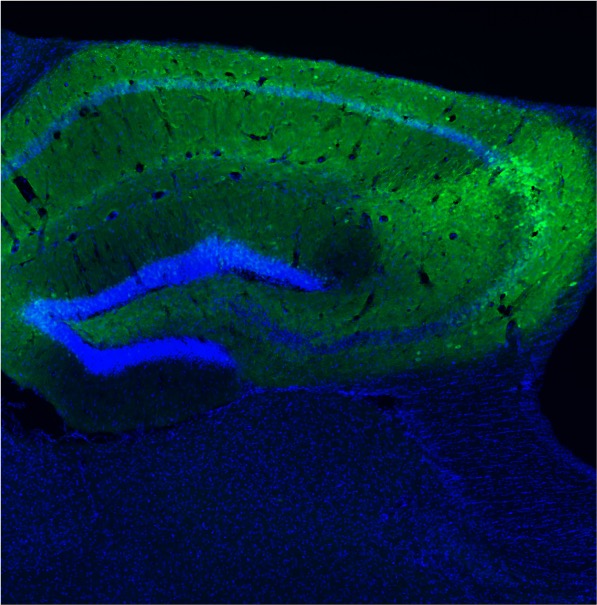
Established in early 2020 (having relocated from Calgary, Alberta, Canada and immediately before the COVID-19 pandemic), the Rho Laboratory at UCSD initially re-established a new line of Kcna1-null knockout mice, and later generated the mouse pilocarpine model. The first-ever rodent video-EEG monitoring suite was also created to enable the gold standard electro-behavioral readout of epileptic seizures. The following are brief summaries of the current major research projects being pursued by laboratory staff:
The Role of Cyclophilin D in the Modulation of Epileptic Activity
Earlier, the Rho Laboratory established a strong mechanistic linkage between the ketone body BHB and anti-seizure effects in epileptic Kcna1-null mice – through inhibition of the mitochondrial permeability transition pore (a multimeric complex spanning both outer and inner mitochondrial membranes and understood to be a critical “death switch” for the cell). Specifically, it was found that beta-hydroxybutyrate interacts with the mitochondrial matrix-localized modulatory protein, cyclophilin D (CypD). The main goal of this project is to elucidate how the ubiquitous mitochondrial protein CypD regulates network excitability and determines cellular viability in the epileptic brain.
Preclinical Validation of Cyclophilin D Antagonists as Novel Anti-Seizure Agents
In collaboration with a United Kingdom-based pharmaceutical company (Cypralis, Ltd.), the Rho Laboratory is investigating the anti-seizure effects of novel cypralide compounds that are selective antagonists of CypD in multiple rodent models of seizures and epilepsy, and examining whether these unique agents can mitigate the inherent interneuronopathy that occurs in the hippocampus of epileptic Kcna1-null mice. The major goal of this project is to generate sufficient pre-clinical and mechanistic data to support the later development of one or more of these compounds for clinical use. Successful validation of a selective CypD antagonist in the epilepsy field would yield the first-ever anti-seizure medication whose primary molecular target would be a mitochondrial protein. This would also represent a successful convergence of neuronal membrane hyperexcitability/hypersynchrony and metabolic intervention at a mitochondrial level.
The Effects of Beta-Hydroxybutyrate on Mitochondrial Sirtuins
BHB is not only a bioenergetic substrate (a product of fatty acid oxidation and used under conditions of fasting or glucose deprivation to produce ATP), but is also a signaling molecule and epigenetic modulator. While BHB has been demonstrated to be a histone deacetylase (HDAC) inhibitor, it is unclear whether this ketone body (as the major metabolite elaborated during treatment with the KD) has other broad epigenetic actions that might affect mitochondria. In this project, the effects of BHB on mitochondrial sirtuins are being explored, particularly in the context of the epileptic brain.
Ketogenic Diet- and Ketone-Mediated Effects on Mitochondrial Dynamics
UCSD boasts only one of three advanced ultra-resolution microscopes in the world capable of imaging live cells and tissues at nanometer resolution, in three dimensions, and in real-time. The AO-LLSM (adaptive optics lattice light sheet microscope), recently installed in the laboratory of Johannes Schoeneberg, PhD (Department of Pharmacology) is being used collaboratively to image mitochondria in hippocampal tissue from epileptic and control mice. In this project, the central hypothesis is that the ketogenic diet and beta-hydroxybutyrate can restore impairments in mitochondrial dynamics (i.e., abnormal fission/fusion processes), thereby improving mitochondrial function.
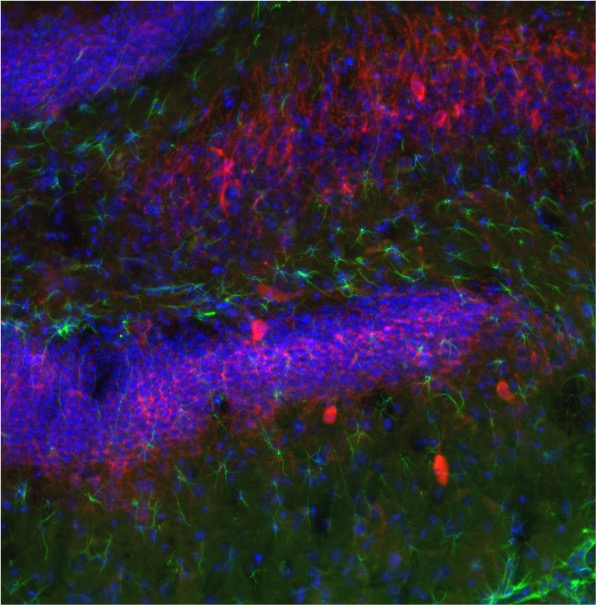
Metabolic Regulation of Astrocytic Potassium Channels in Epileptic Brain
Recently, astrocytic ion channels have been implicated in seizure genesis in both animal models and surgically-resected human epileptic tissues. A principal function of astrocytes is potassium buffering which is essential for normal neuronal activity. Impairment of potassium buffering results in an increase in the extracellular potassium concentration which then provokes neuronal excitability. This project is aimed at determining whether the beneficial effects of the KD and BHB are mediated in part by metabolic regulation of astrocytic potassium channels. To date, basic-translational studies aimed at elucidating underlying mechanisms of KD action have focused more on synaptic mechanisms and neuronal physiology than on neuronal-glial interactions, and the results of the current studies will strengthen the view that neurometabolic function and dysfunction needs to also be considered in the context of mechanistic interactions between astrocytes and neurons.