Research & Trials
- Participate in a Clinical Trial
- Brain Bank
- Clinical Studies Explained
A clinical study involves research using human volunteers (also called participants) that is intended to add to medical knowledge. There are two main types of clinical studies: clinical trials and observational studies.
In a clinical trial (also called an interventional study), participants receive specific interventions according to the research plan or protocol created by the investigators. These interventions may be medical products, such as drugs or devices; procedures; or changes to participants' behavior, for example, diet. Clinical trials may compare a new medical approach to a standard one that is already available or to a placebo that contains no active ingredients or to no intervention. Some clinical trials compare interventions that are already available to each other. When a new product or approach is being studied, it is not usually known whether it will be helpful, harmful, or no different than available alternatives (including no intervention). The investigators try to determine the safety and efficacy of the intervention by measuring certain outcomes in the participants. For example, investigators may give a drug or treatment to participants who have high blood pressure to see whether their blood pressure decreases.
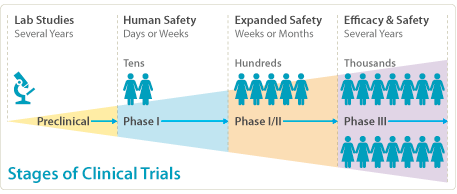
Clinical trials of new medicines or medical devices are done in phases. These phases have different purposes and help researchers answer different questions. For example, phase I clinical trials test new treatments in small groups of people for safety and side effects. Phase II clinical trials look at how well treatments work and further review these treatments for safety. Phase III clinical trials use larger groups of people to confirm how well treatments work, further examine side effects, and compare new treatments with other available treatments.
In an observational study, investigators assess health outcomes in groups of participants according to a protocol or research plan. Participants may receive interventions, which can include medical products, such as drugs or devices, or procedures as part of their routine medical care, but participants are not assigned to specific interventions by the investigator (as in a clinical trial). For example, investigators may observe a group of older adults to learn more about the effects of different lifestyles on cardiac health. For more information regarding clinical studies, please visit the ClinicalTrials.gov Clinical Studies Overview page.
To see a list of our current clinical trials and observational studies, please visit our Participate in Clinical a Trial page.