NIL Projects
Multicolor (non-degenerate) 2-photo excitation
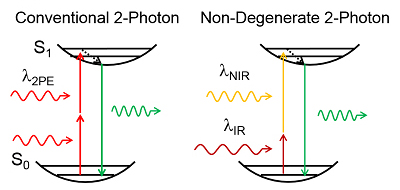 Non-degenerate 2-photon excitation (ND-2PE) uses two independently controlled pulsed laser sources of different photon energies, or color. The first beam is tuned within the standard near infrared (NIR) range of excitation wavelengths used in degenerate 2-photon microscopy. The second laser is tuned to infrared (IR) wavelengths used in 3-photon excitation (3PE). ND-2PE provides a superior alternative to 3-photon microscopy by circumventing low 3PE cross-section while taking advantage of low scattering IR illumination. Efficiency of excitation can be further increased through (1) a systematic search for the most efficient combination of excitation wavelengths for any particular fluorophore and (2) implementation of AO to optimize the overlap of the two focal spots in scattering medium. Finally, the background (“out-of-focus”) excitation can be reduced by spatial displacement of the beams. This project is focused on the development on ND-2PE microscopy instrumentation and application of this technology to achieve deep imaging in the mouse brain.
Non-degenerate 2-photon excitation (ND-2PE) uses two independently controlled pulsed laser sources of different photon energies, or color. The first beam is tuned within the standard near infrared (NIR) range of excitation wavelengths used in degenerate 2-photon microscopy. The second laser is tuned to infrared (IR) wavelengths used in 3-photon excitation (3PE). ND-2PE provides a superior alternative to 3-photon microscopy by circumventing low 3PE cross-section while taking advantage of low scattering IR illumination. Efficiency of excitation can be further increased through (1) a systematic search for the most efficient combination of excitation wavelengths for any particular fluorophore and (2) implementation of AO to optimize the overlap of the two focal spots in scattering medium. Finally, the background (“out-of-focus”) excitation can be reduced by spatial displacement of the beams. This project is focused on the development on ND-2PE microscopy instrumentation and application of this technology to achieve deep imaging in the mouse brain.
Physiological basis of non-invasive human imaging signals
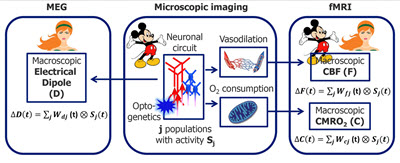 The overarching idea is that activation of different neuronal cell types has different signatures in the evoked CBF (ΔF), CMRO2 (ΔC), and the macroscopic current dipole responses (ΔD), and by measuring these responses noninvasively in the human brain we will be able to probe more deeply the underlying cellular and circuit activity.
The overarching idea is that activation of different neuronal cell types has different signatures in the evoked CBF (ΔF), CMRO2 (ΔC), and the macroscopic current dipole responses (ΔD), and by measuring these responses noninvasively in the human brain we will be able to probe more deeply the underlying cellular and circuit activity.For each neuronal population (e.g., excitatory and inhibitory), the current dipole moment (measured by MEG) and CBF/CMRO2 (measured by calibrated BOLD) can be expressed as convolution of the neuronal activity for neuronal population of cell type j with time-resolved weighting factor W(t) for that cell type (that can be thought of as cell-type-specific impulse response function, IRF). The weighting factors W(t) will be determined experimentally using selective optogenetic activation of specific cell types in mice. Read more on PubMed.gov.
This project combines data acquisition in mice and humans with computational modeling.
In vivo 2-photon imaging of O2 availability
Brain activity largely relies on mitochondrial oxidative metabolism to meet the energy demands. Therefore, the oxygenation level of cerebral tissue is one of the most basic physiological parameters. As such, it can be used as a biomarker in animal models of neurodegenerative disease, either to detect a departure from normal physiology or as an objective criterion for the effectiveness of treatment. However, our ability to probe microscopic availability of O2 during different levels of neuronal activity has been limited to point measurements using O2 electrodes. Recently, in collaboration with Drs. Sava Sakadzic and David Boas, we developed a new technology for in vivo measurements of pO2. This new method is based on 2-photon imaging of phosphorescent lifetime of O2-sensitive probe PtP-C343, developed by our collaborator Dr. Sergey Vinogradov, and allows measurements of intravascular or interstitial pO2 with an unprecedented spatial resolution and is well suited for imaging of pO2 changes during functional activation. We utilize this new technology to investigate dependence of pO2 changes on the location relative to the 3-D vascular network and center of neuronal response.
Check out our new paper on estimation of Cerebral Metabolic Rate of O2 (CMRO2) using this technology.
Cellular and molecular mechanisms of neurovascular communication
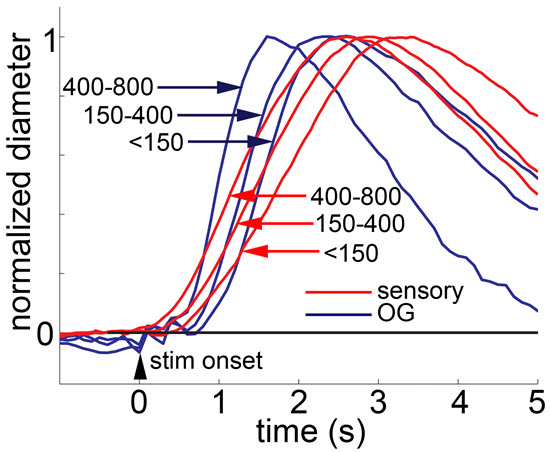
Mechanistic understanding of microscopic processes that govern regulation of cerebral blood flow and metabolism is a critical component for laying a solid physiological foundation for interpreting functional neuroimaging studies and a prerequisite for development of targeted treatments in neurovascular disease. A growing body of experimental evidence, including our own, indicates that while molecules produced by increased energy metabolism do have a vasoactive effect, much of the acute cerebral blood flow response in vivo under healthy conditions is driven by vasoactive messengers related to neuronal signaling. This project combines advanced in vivo 2-photon imaging technology with optogenetics and pharmacological manipulations in the mouse cortex to identify cellular players and signaling molecules in the context of functional hyperemia.
Check out our recent publication here.
Functional imaging of human iPSCs-derived neurons integrated in mouse cortex
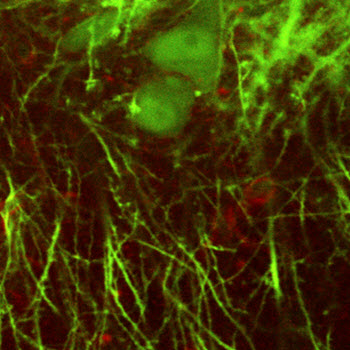 Recent advances in stem cell technology now allow generation of neurons from human cells isolated form peripheral tissues (e.g., skin) opening unprecedented opportunities for investigation of human brain disease linked to known genetic variations. Using this technology, the first step is to reprogram patient’s cells to an early developmental state producing pluripotent stem cells (iPSCs). Next, iPSCs can be differentiated into desired neuronal subtypes, e.g., glutamatergic or GABAergic, potentially creating neuronal networks in a dish. However, the lack of the natural brain microenvironment in a dish can influence the phenotype and maturation. One potential strategy to overcome these limitations is transplantation of iPSC-derived neuronal precursor cells into the mouse brain. In collaboration with Alysson Muotri, we use in vivo imaging to evaluate integration of human neurons into the local mouse circuits. This experimental approch has a potential to serve as a translational tool for studying disease mechanisms and evaluating the impact of candidate drugs.
Recent advances in stem cell technology now allow generation of neurons from human cells isolated form peripheral tissues (e.g., skin) opening unprecedented opportunities for investigation of human brain disease linked to known genetic variations. Using this technology, the first step is to reprogram patient’s cells to an early developmental state producing pluripotent stem cells (iPSCs). Next, iPSCs can be differentiated into desired neuronal subtypes, e.g., glutamatergic or GABAergic, potentially creating neuronal networks in a dish. However, the lack of the natural brain microenvironment in a dish can influence the phenotype and maturation. One potential strategy to overcome these limitations is transplantation of iPSC-derived neuronal precursor cells into the mouse brain. In collaboration with Alysson Muotri, we use in vivo imaging to evaluate integration of human neurons into the local mouse circuits. This experimental approch has a potential to serve as a translational tool for studying disease mechanisms and evaluating the impact of candidate drugs.
Quantitative imaging of calcium dynamics with 2-photon resolution
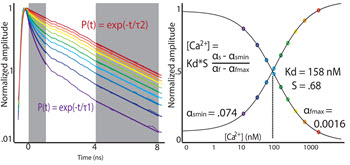
Intracellular concentration of calcium ions is a key parameter in neuronal physiology, intimately related to cell’s regulatory mechanisms and gene expression. Therefore, in healthy neurons, multiple mechanisms ensure fast restoration of calcium concentration in the cytosol following dynamic signaling events. To enable imaging of these processes, we implement fluorescent lifetime imaging microscopy (FLIM) for quantitative estimation of intracellular calcium concentration in vivo and in vitro with 2-photon resolution. Further, we apply FLIM to evaluate departures from normal calcium homeostasis in mouse models of brain disease.
In vivo microscopic biomarkers of neurodegeneration
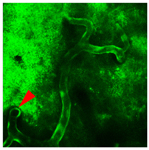 The major goal of this project is to establish a defined set of in vivo imaging functional markers characterizing the progression of neuro-glio-vascular pathology in Alzheimer’s and Parkinson’s Disease in transgenic mouse models. We use 2-photon microscopy for in vivo imaging of calcium homeostasis and the functional interactions among the capillaries, glia, and neurons, termed the "neurovascular unit” (NVU). The in vivo NVU approach provides a more integrative answer to neurological damage which may be closer to modeling the clinical reality. The lessons learned from in vivo NVU imaging may also contribute to understanding of other neurodegenerative disorders, like multi-infarct dementia, frontotemporal dementia, amyotrophic lateral sclerosis and trinucleotide repeat disorders, in which a deterioration of neurovascular physiology involving alterations in intracellular calcium homeostasis contributes to motor and cognitive decline.
The major goal of this project is to establish a defined set of in vivo imaging functional markers characterizing the progression of neuro-glio-vascular pathology in Alzheimer’s and Parkinson’s Disease in transgenic mouse models. We use 2-photon microscopy for in vivo imaging of calcium homeostasis and the functional interactions among the capillaries, glia, and neurons, termed the "neurovascular unit” (NVU). The in vivo NVU approach provides a more integrative answer to neurological damage which may be closer to modeling the clinical reality. The lessons learned from in vivo NVU imaging may also contribute to understanding of other neurodegenerative disorders, like multi-infarct dementia, frontotemporal dementia, amyotrophic lateral sclerosis and trinucleotide repeat disorders, in which a deterioration of neurovascular physiology involving alterations in intracellular calcium homeostasis contributes to motor and cognitive decline.
Hemodynamic modeling in realistic microvascular networks
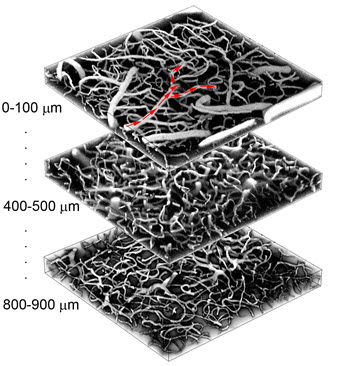 In collaboration with David Boas from Harvard Medical School, we develop a dynamic mechanistic vascular model, which incorporates biophysically realistic parameters at the microscopic scale and is embedded in a realistic geometry derived from 2-photon measurements of an in vivo vascular network. The activity of specific neurons cause dynamic changes in the diameter of the arterioles/capillaries, resulting in CBF changes due to the altered vascular resistance due to these vessel diameter changes. The model also incorporates oxygen dynamics, which enables estimation of intra- and extravascular microscopic oxygenation changes.
In collaboration with David Boas from Harvard Medical School, we develop a dynamic mechanistic vascular model, which incorporates biophysically realistic parameters at the microscopic scale and is embedded in a realistic geometry derived from 2-photon measurements of an in vivo vascular network. The activity of specific neurons cause dynamic changes in the diameter of the arterioles/capillaries, resulting in CBF changes due to the altered vascular resistance due to these vessel diameter changes. The model also incorporates oxygen dynamics, which enables estimation of intra- and extravascular microscopic oxygenation changes.
Modeling of neuronal populations
 In collaboration with Gaute Einevoll from the Norwegian University of Life Sciences, this project is focused on statistical and mechanistic modeling of neuronal circuits using in vivo and in vitro electrophysiological data and cell reconstructions. The overall goal of this project is data-driven development of network models with a specific focus on the phenomenon of surround and transcallosal inhibition as an emergent property of neuronal circuits in the somatosensory cortex. Specifically, we develop a set of interconnected models at different levels of detail, from simplified spiking models to firing-rate population models of cortical columns containing different cell types across cortical layers. Using a joint experimental and modeling effort we will dissect the mechanisms that underlie the evolution of surround and transcallosal inhibition arising from differential layer-specific recruitment of excitatory and multiple types of inhibitory neurons. Eventually, the neuronal and vascular models will be joined into a neurovascular model that would predict the hemodynamic response given neuronal activity in cell-type specific populations.
In collaboration with Gaute Einevoll from the Norwegian University of Life Sciences, this project is focused on statistical and mechanistic modeling of neuronal circuits using in vivo and in vitro electrophysiological data and cell reconstructions. The overall goal of this project is data-driven development of network models with a specific focus on the phenomenon of surround and transcallosal inhibition as an emergent property of neuronal circuits in the somatosensory cortex. Specifically, we develop a set of interconnected models at different levels of detail, from simplified spiking models to firing-rate population models of cortical columns containing different cell types across cortical layers. Using a joint experimental and modeling effort we will dissect the mechanisms that underlie the evolution of surround and transcallosal inhibition arising from differential layer-specific recruitment of excitatory and multiple types of inhibitory neurons. Eventually, the neuronal and vascular models will be joined into a neurovascular model that would predict the hemodynamic response given neuronal activity in cell-type specific populations.
In vivo 2-photon imaging of cellular metabolism
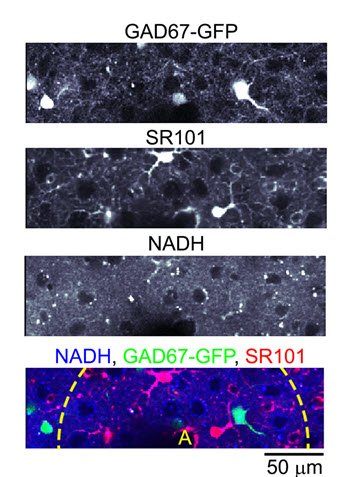 Intravital imaging of cell-specific metabolic activity is of key importance for understanding of a wide range of clinical conditions. Among them is compromised blood perfusion following a stroke and a decrease in efficiency of single-cell respiratory processes that occurs in neurodegenerative diseases such as Alzheimer's and Parkinson's disease. However, no methods are available today for in vivo measurement of single cell metabolism with sufficient sensitivity to resolve fast metabolic events related to ongoing neuronal electrical activity and neuronal responses to stimulation. To meet this challenge, we will adapt an existing technology, 2-photon laser scanning microscopy, for high-resolution microscopic imaging of functional metabolism of single brain cells, taking advantage of intrinsic fluorescence of metabolic cofactor β-nicotinamide adenine dinucleotide (NADH). Specifically, we address the relative contribution of oxidative phosphorylation and glycolysis to the transient metabolic response in neurons and astrocytes, test the relationship between neuronal/astrocytic metabolic response and regulation of blood flow, and work towards establishing functional NADH imaging as a biomarker for hypoxia and mitochondrial dysfunction.
Intravital imaging of cell-specific metabolic activity is of key importance for understanding of a wide range of clinical conditions. Among them is compromised blood perfusion following a stroke and a decrease in efficiency of single-cell respiratory processes that occurs in neurodegenerative diseases such as Alzheimer's and Parkinson's disease. However, no methods are available today for in vivo measurement of single cell metabolism with sufficient sensitivity to resolve fast metabolic events related to ongoing neuronal electrical activity and neuronal responses to stimulation. To meet this challenge, we will adapt an existing technology, 2-photon laser scanning microscopy, for high-resolution microscopic imaging of functional metabolism of single brain cells, taking advantage of intrinsic fluorescence of metabolic cofactor β-nicotinamide adenine dinucleotide (NADH). Specifically, we address the relative contribution of oxidative phosphorylation and glycolysis to the transient metabolic response in neurons and astrocytes, test the relationship between neuronal/astrocytic metabolic response and regulation of blood flow, and work towards establishing functional NADH imaging as a biomarker for hypoxia and mitochondrial dysfunction.
